Risdiplam (brand name Evrysdi) is a medication used to treat spinal muscular atrophy (SMA). It is a prescription medicine approved by the US Food and Drug Administration (FDA) to treat SMA types 1, 2, 3, and 4. This comprehensive guide will provide information on the uses, benefits, side effects, and cost of Risdiplam. It will also include information on how to cover Risdiplam cost in India through online crowdfunding, and Risdiplam uses to treat SMA. Finally, this guide will provide resources for finding support and assistance accessing Risdiplam.
Read More: Impactguru hospital finder
Table of Contents
- How Much Does Risdiplam Cost In India?
- Risdiplam Uses & Side Effects
- Which SMA Treatment Is More Effective- Risdiplam Or Zolgensma?
- Preventive Measures To Be Taken Before And After Consuming Risdiplam
- How Effective Is Risdiplam In Treating SMA?
- Is Risdiplam Approved In India?
- What Is Risdiplam?
- For Which Rare Disease Is Risdiplam (Evrysdi) Used As A Medication Therapy?
- How Risdiplam Stops The Progression Of SMA?
- How Is Risdiplam Taken?
- History Of Risdiplam
- Financial Options To Cover The Cost Of Risiplam (Evrysdi) In India
- Medical Crowdfunding- An Effective Financial Solution For Medical Cause
How Much Does Risdiplam Cost In India?

Risdiplam is a medication used to treat spinal muscular atrophy (SMA). It is a prescription drug approved in India by the CDSCO (Central Drugs Standard Control Organization) on the recommendation of the Drugs Controller General of India (DCGI). One bottle of Risdiplam costs about Rs 6 lakh, with a dosage of 0.75 mg/ml powder, and it’s available for oral solution. It is available in a 30-mg tablet form and is to be taken orally. The dosage of Risdiplam may vary depending on the patient’s needs and must be prescribed by a qualified healthcare professional. The price of Risdiplam in India may vary depending on the pharmacy where it is purchased.
Risdiplam is a prescription medication for treating spinal muscular atrophy (SMA) in people with type 1, 2, or 3 SMA. While the drug is expensive, it is one of the three drugs approved to treat SMA and is proven to slow the progression of the disease in affected individuals. Due to the lack of other treatments available, Risdiplam’s high price is justified. The high cost of the drug is also due to the cost of research and development and the cost of manufacturing the medication.
Why Is Risdiplam So Expensive?
Risdiplam’s high price is due to several factors. Firstly, the drug is a breakthrough therapy for a rare and life-threatening neuromuscular disorder called spinal muscular atrophy (SMA). This makes it a highly sought-after treatment option. Secondly, the drug is complex and expensive to produce, and its formulation is tailored for each patient, further driving up the cost. Lastly, the limited availability of the drug, due to its orphan drug status, means that only a few manufacturers can produce it, thus limiting competition and driving up prices.
Reasons For The High Price Of Risdiplam In India
Risdiplam’s price is so high in India because it is an expensive and innovative medication used to treat spinal muscular atrophy (SMA). As a new and potentially life-changing drug, it is costly to produce, and the cost is passed on to consumers. In addition, the Indian government has implemented price control mechanisms, making it difficult to reduce the cost of the drug. Furthermore, the drug is only available through prescription, and the Indian healthcare system has limited access to specialists who are qualified to prescribe the medication. All of these factors contribute to the high price of Risdiplam in India.
Additionally, the cost of Risdiplam will likely decrease over time as more healthcare providers begin to cover it.
Some Of The Ways To Decrease Risdiplam Price In India
1. Increase the number of suppliers: One of the primary ways to reduce the cost of Risdiplam in India is to increase the number of suppliers in the market. This will create competition among the suppliers, allowing them to lower their prices to attract more customers. Additionally, with more suppliers in the market, the cost of raw materials and manufacturing processes can be reduced, resulting in lower prices for the final product.
2. Negotiate with the manufacturers: The Indian government should negotiate with the manufacturers of Risdiplam to reduce the cost of the drug. This can be done by offering incentives such as tax breaks or subsidies to encourage the manufacturers to lower the cost of the medicine. Additionally, the government can mandate the manufacturers to reduce the cost of the drug.
3. Bulk purchases: Bulk purchases of Risdiplam by the government or other healthcare organizations can help reduce the cost of the drug. By buying in bulk, the government or healthcare organizations can negotiate a lower price with the manufacturers, resulting in a more affordable drug for the patients.
4. Provide subsidies to patients: The government can also provide grants to patients to help reduce the cost of Risdiplam. This will ensure that the drug is accessible to everyone, regardless of their financial status.
5. Implement Price Control Mechanisms: Price control mechanisms, such as price ceilings or floors, can also be implemented to ensure the drug is made available at an affordable price. These mechanisms will help ensure that the drug is not sold at an exorbitant price.
6. Increase Awareness: Increasing awareness about the availability of Risdiplam and its benefits can help reduce the cost of the drug. By increasing the demand for the drug, the manufacturers will be encouraged to lower the price to attract more customers. Additionally, more people will be made aware of the availability of the drug, making it easier for them to access the medication.
Risdiplam Uses & Side Effects

It is the first therapy to be approved for this condition. Risdiplam is a small molecule taken orally and works by increasing the amount of a protein called survival of motor neuron 1 (SMN1), which is essential for developing and maintaining muscle function. It is believed to positively affect nerve cells, allowing them to communicate better with each other and sustain muscle function.
Risdiplam treats spinal muscular atrophy (SMA) in patients of all ages. It works by increasing the Survival Motor Neuron 1 (SMN1) protein. This protein is essential for the development and maintenance of muscle function. By increasing the amount of this protein, Risdiplam helps to restore and maintain muscle function.
Risdiplam has also been studied in clinical trials to treat SMA-related respiratory impairment. Studies have shown that Risdiplam can improve breathing capacity in people with SMA and their quality of life.
Risdiplam has also been studied in clinical trials for its potential to treat other neurological conditions, such as amyotrophic lateral sclerosis (ALS) and Huntington’s disease. Although the results of these studies are still preliminary, and more research is needed to confirm the efficacy of Risdiplam for these conditions, the findings indicate that Risdiplam may potentially treat a range of neurological conditions.
Overall, Risdiplam is an important new treatment option for spinal muscular atrophy that has the potential to better the lives of SMA patients and their families. It is also being observed as a potential treatment for other neurological conditions, and further research will help determine its efficacy.
What Are The Side-effects Of Risdiplam?
Risdiplam is a drug used to treat SMA in adults and children two months and older. A Survival Motor Neuron 2 (SMN2) splicing modifier helps increase the full-length SMN protein in the body.
Common side effects of Risdiplam include:
- Gastrointestinal (GI) issues such as diarrhea, constipation, nausea, and vomiting
- Respiratory issues such as shortness of breath, cough, and respiratory infections
- Fatigue
- Headache
- Muscle spasms
- Flu-like symptoms such as fever and chills
- Joint pain
- Increased appetite
- Rashes
- Low blood cell counts (neutropenia, lymphopenia, and thrombocytopenia)
- Abnormal liver function tests
- Elevations in certain blood sugar levels
- Elevations in certain cholesterol levels
- Increased uric acid levels
In addition, Risdiplam may cause an increased risk of infection, certain types of cancer, and an increased risk of developing autoimmune diseases. Patients should consult their doctor if they experience these side effects while taking Risdiplam.
Which SMA Treatment Is More Effective- Risdiplam Or Zolgensma?
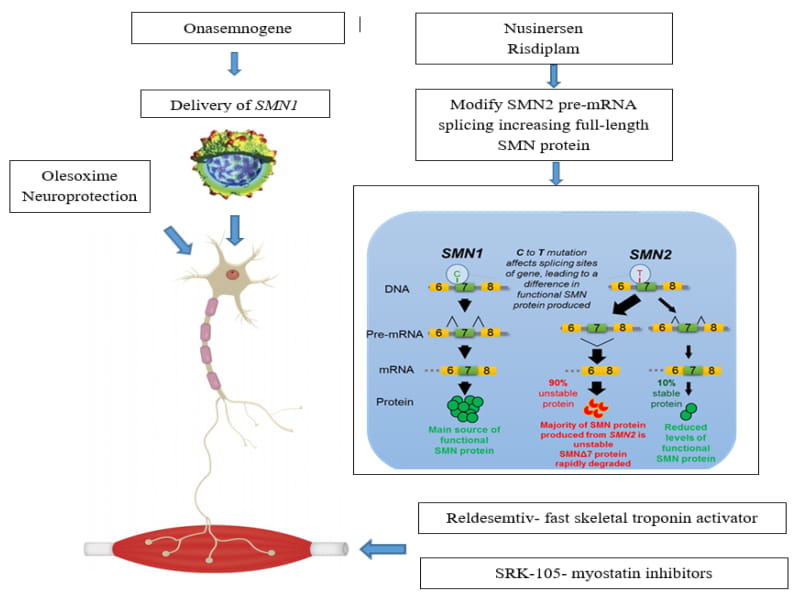
Risdiplam and Zolgensma are both treatments for Spinal Muscular Atrophy (SMA), a genetic disorder that affects the nervous system and causes muscle weakness. The FDA has approved both treatments, which are effective in treating the disease.
- Risdiplam is an oral medication designed to increase the amount of SMN protein in the body. It works by increasing the expression of a gene called SMN2, which is responsible for producing SMN protein. It can be taken daily for an extended period and is generally well-tolerated.
- Zolgensma is a gene therapy where it uses a modified virus to deliver a functioning copy of the SMN1 gene to the body’s cells. Zolgensma price in india It is administered as a one-time intravenous infusion and has been effective in treating SMA in children under two.
In terms of effectiveness, both are effective in treating SMA. However, zolgensma available in india may be more effective in treating the most severe forms of SMA, as it can be administered as a one-time treatment. Risdiplam effectively treats milder forms of SMA and can be taken daily for an extended time.
Ultimately, the best treatment for an individual will depend on their SMA severity and medical needs. It is crucial to speak with a healthcare provider to determine which treatment may be best for you or your child.
Preventive Measures To Be Taken Before And After Consuming Risdiplam
Before Consuming Risdiplam:
1. Consult your doctor and get tested for the SMA gene mutation. Risdiplam is only effective in people with a specific gene mutation that causes SMA, so it is vital to get tested before consuming the drug is essential.
2. Discuss the potential side effects of Risdiplam with your doctor. Like any other medication, Risdiplam may cause some side effects. Discussing these side effects with your doctor before taking the drug is important.
3. Have an entire physical and blood work done before taking Risdiplam. This will help your doctor monitor your progress and adjust the dosage if necessary.
4. Ask your doctor about any dietary or lifestyle changes necessary to take Risdiplam.
After Consuming Risdiplam:
1. Monitor your progress closely. Keep track of changes in your physical and cognitive abilities, and report any changes to your doctor.
2. Report any new or worsening side effects.
3. Maintain a healthy diet and lifestyle. Exercise and eat a balanced diet to ensure Risdiplam is as effective as possible.
4. Take your medication as prescribed. Do not change your dosage or stop taking your medication without consulting your doctor.
5. Follow up with your doctor regularly. Get regular check-ups and blood work done to ensure Risdiplam works as it should.
How Effective Is Risdiplam In Treating SMA?

Risdiplam is a drug developed to treat Spinal Muscular Atrophy (SMA). It works by improving the amount of the body’s Survival Motor Neuron (SMN) protein, which is essential for muscle function. The SMN protein is produced by a gene known as the SMN1 gene. People with SMA have a mutation in this gene, so they don’t make enough of the SMN protein. Risdiplam works by restoring the function of the SMN gene, allowing it to produce more SMN protein.
Risdiplam has been found to be effective in treating SMA in both children and adults. In clinical trials, Risdiplam was found to increase the amount of SMN protein in the body and improve muscle strength, motor function, and quality of life. In a study of 2-5-year-old children with SMA, Risdiplam significantly improved motor function and reduced the risk of disease progression. In another study of adults with SMA, Risdiplam improved muscle strength, motor function, and quality of life and reduced the risk of disease progression.
Risdiplam is an effective treatment for SMA and has been shown to improve muscle strength, motor function, and quality of life. It can also reduce the risk of disease progression and can be used to treat both children and adults with SMA.
Is Risdiplam Approved In India?
Risdiplam, also known as Evrysdi, has been approved in India for treating Spinal Muscular Atrophy (SMA). Roche, the Swiss drug company, launched Evrysdi in India in July 2021. It is the first and only approved medication for SMA in India. SMA is a rare neuromuscular disease and is India’s leading genetic cause of infant mortality, affecting approximately one in 7,744 live births. Until now, Spinraza and Zolgensma have been the only two gene therapies available in the country. However, Evrysdi is cheaper and requires no hospitalization or specialized care center. Roche also plans to offer the drug to patients in India at a discounted price and under the patient support program, providing free bottles based on purchases made by patients.
Patients who weigh over 20 kg would need to pay around Rs 72 lakh for a full year of treatment during the first two years with the patient support program. Roche has launched a patient support program that provides three bottles of the drug-free for every two bottles bought in the first two years of treatment and two bottles for every one purchased from the third year onwards.
What Is Risdiplam?

Risdiplam is a drug being developed to treat people with Spinal Muscular Atrophy (SMA). SMA is a type of rare genetic disease that leads to progressive muscle weakness and loss of movement. Risdiplam is a small molecule designed to increase levels of the SMN protein, which is essential for motor neuron survival. It can improve motor, muscle strength, and respiratory function in people with SMA.
Risdiplam is a medication that Roche and PTC Therapeutics, Inc. developed to treat spinal muscular atrophy (SMA). SMA is a rare, genetic neuromuscular disorder that affects the ability of muscles to function properly. A mutation in the SMN1 gene causes it and can lead to muscle weakness, breathing difficulties, and a shortened lifespan. Risdiplam is an oral medication that increases the amount of functional SMN protein in the body. This helps improve muscle function and reduce the severity of SMA symptoms.
Risdiplam is a small molecule that can be taken orally, meaning it does not have to be injected and has undergone clinical trials to demonstrate its safety and efficacy. It has been tested in adults and children with SMA and has improved motor function, quality of life, and survival in those with SMA. Risdiplam has been approved by the U.S. Food and Drug Administration (FDA) to treat SMA in individuals two months of age and older.
Risdiplam is the first oral medication officially approved for treating SMA. It can potentially improve the lives of individuals with SMA and is an essential breakthrough in treating this rare disorder.
For Which Rare Disease Is Risdiplam (Evrysdi) Used As A Medication Therapy?
Risdiplam (brand name Evrysdi) is a medication for treating a rare genetic disorder called Spinal Muscular Atrophy (SMA). It is a progressive, genetic neuromuscular disorder that causes muscle weakness and degeneration and can eventually lead to paralysis and death. Risdiplam is a small molecule designed to increase protein levels called survival motor neuron 2 (SMN2). This protein helps keep motor neurons alive and functioning, and when it is absent or low, nerve cells die, and symptoms of SMA appear.
By increasing SMN2 protein production, Risdiplam helps improve muscle strength and motor function in people with SMA. It can be used to treat all types of SMA, including type 1, type 2, type 3, and type 4. The medication is given as a liquid taken by mouth twice daily. It is most effective when taken early in the disease, so starting treatment as soon as possible is essential.
Risdiplam has been approved for use in the United States by the Food and Drug Administration (FDA) in the United States and is available through a REMS (Risk Evaluation and Mitigation Strategy) program. The REMS program ensures that the medication is prescribed and used appropriately. In addition, patients must be monitored very closely for any side effects, such as difficulty breathing or swallowing, chest pain, diarrhea, or an irregular heartbeat.
How Risdiplam Stops The Progression Of SMA?
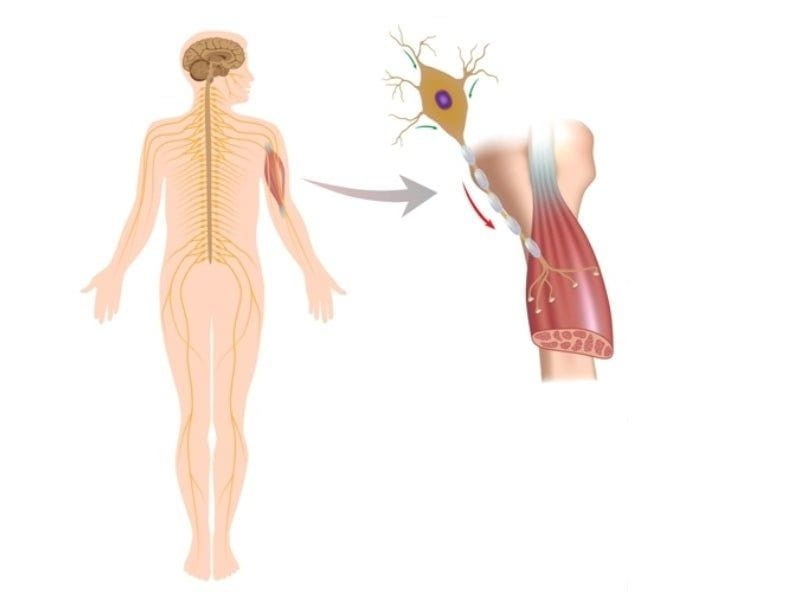
Risdiplam is a small molecule drug that increases the Survival Motor Neuron (SMN) protein. SMN protein is essential for adequately functioning motor neurons responsible for controlling muscle movement. With too little SMN protein, the motor neurons cannot function properly, leading to muscle weakness seen in SMA.
Risdiplam works by binding to a specific site on the SMN2 gene, which is a gene related to the SMN protein. This binding activates the gene, causing it to produce more SMN protein. By increasing the amount of SMN protein in the body, Risdiplam can stop the progression of SMA. In addition to its ability to stop the progression of SMA, Risdiplam also helps to improve muscle strength, coordination, and endurance in those with SMA and improve the quality of life for those living with SMA.
How Is Risdiplam Taken?
Risdiplam is an oral liquid medicine that is taken daily by mouth. It comes in two forms: a pre-measured single-dose ampoule or a pre-measured single-dose vial.
- The ampoule is the most convenient form of Risdiplam. The user simply pulls off the top of the ampoule and squeezes the contents into the mouth. The ampoule should be consumed within 30 minutes after opening and should not be stored or refrigerated.
- The vial form of Risdiplam is also easy to use. The user must first remove the cap from the vial, then draw the prescribed dose of medicine with a syringe. The user should then place the syringe tip in the mouth and slowly push the plunger to administer the dose. The medicine should be swallowed whole, not chewed or crushed. The vial should be discarded after use and should not be stored or refrigerated.
It is essential to take Risdiplam precisely as prescribed by your doctor and take it simultaneously each day. If any of the doses are missed, they should be consumed as soon as possible, but not if it is close to the next scheduled dose. Taking more than the prescribed dose can cause serious side effects.
It is vital to follow your doctor’s instructions carefully when taking Risdiplam. Your doctor may also monitor your response to the medicine and adjust the dose accordingly. It is essential to contact your doctor immediately if you experience any side effects or if your symptoms do not improve.
History Of Risdiplam
Risdiplam (brand name Evrysdi) is a medication developed by Roche, formerly PTC Therapeutics, for treating spinal muscular atrophy (SMA). It was approved by the US Food and Drug Administration (FDA) in August 2020.
Risdiplam’s development began in 2011 when PTC Therapeutics acquired the rights to develop a potential drug for SMA from the University of Massachusetts Medical School. Research and development of Risdiplam were initially funded by PTC, as well as by the Organizations like NINDS (National Institute of Neurological Disorders and Stroke) and the SMA Foundation.
The development of Risdiplam began in 2011 when PTC Therapeutics and Roche collaborated to develop a potential therapy for SMA. In the ensuing years, PTC and Roche conducted clinical trials to study the safety and efficacy of Risdiplam. The trials included multiple Phase III and Phase II studies and an open-label extension study. These trials’ results demonstrated that Risdiplam effectively improved motor function in SMA patients.
The first clinical trials of Risdiplam began in 2015. In the early trials, Risdiplam was tested in patients with Type 1 and Type 2 SMA. After a series of successful trials, the FDA approved Risdiplam for the treatment of SMA in August 2020. The drug is taken orally and is expected to be effective in Type 1 and Type 2 SMA patients. It is also likely to be effective in treating other types of SMA, such as Type 3 and Type 4.
Clinical Trials For Risdiplam
Risdiplam is an investigational drug for treating spinal muscular atrophy (SMA). It is being developed by the pharmaceutical company Roche and the US biotechnology company PTC Therapeutics.
- The clinical trials of Risdiplam began in 2018, with the Phase 1 clinical trial (FIREFISH) designed to assess the safety and tolerability of Risdiplam in infants aged 1 to 7 months with SMA type 1.
- The Phase 2 clinical trial (SUNFISH) is an open-label, multicenter, international, dose-finding study assessing the effectiveness and safety of Risdiplam in children aged 2 to 25 years with SMA type 2 or 3. The trial enrolled 128 participants and is expected to be completed in 2021.
- The Phase 3 clinical trial (JEWELFISH) is an open-label, multicenter, international, placebo-controlled study designed to assess the efficacy and safety of Risdiplam in participants aged 2 to 25 years with SMA type 2 or 3.
- The Phase 4 clinical trial (RAINBOWFISH) is an open-label, multicenter, international study designed to assess the long-term safety, tolerability, and efficacy of Risdiplam in participants aged 2 to 25 years with SMA type 2 or 3.
Indian Health Authorities approved Evrysdi® after reviewing its efficacy and safety data from three global clinical studies designed to represent a broad spectrum of people living with SMA:
- FIREFISH3 in symptomatic infants aged 1 to 7 months with Type 1 SMA
- SUNFISH3 in children and adults aged 2 to 25 years. SUNFISH is the first placebo-controlled trial to include adults with Types 2 and 3 SMA.
The Phase 1 and 2 clinical trial results of Risdiplam have been promising, with participants showing improved motor function and increased survival rates. Phase 3 and 4 clinical trials are ongoing, so the full results are yet to be seen.
Results Of Risdiplam For SMA Treatment
Risdiplam (Evrysdi) is the first oral medication approved for treating spinal muscular atrophy (SMA). SMA is a rare genetic disease affecting muscle strength and movement due to insufficient survival motor neuron (SMN) protein levels caused by a defect in the SMN1 gene. Risdiplam targets SMN2, another gene, to improve the production of viable SMN protein and has been approved in multiple countries worldwide for treating SMA in patients aged ≥ 2 months. Phase 2/3 clinical trials have shown that Risdiplam significantly improves motor function in infants with SMA type 1 and in patients aged 2-25 years with SMA type 2 or 3, with improvements maintained for up to 2 years of treatment.
Risdiplam was generally well-tolerated and provides a convenient and valuable treatment option across a broad range of patient ages and subtypes of SMA. The drug was effective in two clinical studies involving infants and older patients with SMA, and the most common side effects include fever, diarrhea, and joint pain. Therefore, Risdiplam is an effective treatment option for SMA patients, and it can significantly improve their motor function and overall quality of life.
Financial Options To Cover The Cost Of Risiplam (Evrysdi) In India
Roche, the company that manufactures Evrysdi (Risdiplam), has launched a patient support program that provides patients with free home medicine delivery and discounts on the drug. Additionally, non-profit organizations and patient advocacy groups provide financial assistance programs to help SMA patients access treatment, including Risdiplam. It is also possible that insurance plans or private health coverage may cover some portion of the cost of Risdiplam. It would be best to speak with a healthcare provider or contact Roche for more information on financial assistance options for accessing Risdiplam in India. Another financial option could be medical crowdfunding on a trusted and reliable fundraising platform in India.
Medical Crowdfunding- An Effective Financial Solution For Medical Cause

Medical fundraising on an online crowdfunding platform in India is becoming increasingly popular to access funds for medical treatments and other medical needs. The concept of medical crowdfunding is simple: individuals create a fundraising page on a crowdfunding platform, which allows them to share their stories and ask for donations from their families, friends, and the general public. Donors can then make donations to the campaign, which goes directly to the medical cause.
Medical crowdfunding has become increasingly popular in India due to the increasing cost of medical treatments and the lack of access to affordable healthcare. It has allowed individuals to access funds for treatments that would otherwise be financially out of reach. Medical crowdfunding has also benefited people who cannot access traditional forms of funding, such as loans or grants. This is because crowdfunding does not require an individual to have a good credit score or other proof of income.
Overall, medical crowdfunding on an online crowdfunding platform in India is a great way to access funds for medical treatments and other medical needs. It is convenient and secure and allows individuals to access funds quickly and easily.
How To Raise Funds For Risdiplam?
1. Select a crowdfunding platform- Choose a reliable and secure crowdfunding platform trusted by donors and accessed easily. This platform should have an easy-to-use interface and should provide multiple payment options to donors.
2. Create a campaign page– Set up a page on the platform that provides information about Risdiplam and the need to fund its development in India. Include a clear call to action and a goal for the campaign.
3. Promote the campaign- Utilize social media, email, and other online and offline platforms to spread the word about the campaign. Ask your friends and family to share the campaign and encourage them to donate.
4. Ask for donations- Reach out to individuals, businesses, and organizations that may be willing to donate or help promote the campaign.
5. Monitor the campaign’s progress– Track the campaign’s progress and keep donors updated.
6. Utilize local resources- Reach out to local organizations and businesses that may be willing to help fund the campaign.
7. Thank donors– Show your appreciation to donors by thanking them for their support and providing updates on the campaign’s progress.
Try ImpactGuru To Raise Funds For Risdiplam (Evrysdi)
To cover the cost of Risdiplam in India with a crowdfunding platform, you can start by creating a campaign on a trusted crowdfunding platform like Impact Guru. You can share your story, medical condition, and why you need Risdiplam. You can also mention the cost of the drug, which is approximately INR 6,00,000 ($8,000) per month. You can set a fundraising goal and share the campaign on social media and with your friends and family.
Fundraising for the first time may seem challenging, but thousands of people have successfully raised funds for their medical cause at ImpactGuru, for high as 18 crores to the lowest amount. Anyone in need can start a fundraiser in just 5 minutes at ImpactGuru. You can also receive 24*7 customer support for a hassle-free fundraiser journey.
Start your fundraiser today and focus more on your SMA care or treatment than on ways to cover the cost of Risdiplam in India.












