Table of Contents
- Duchenne Muscular Dystrophy and Its Long Road to Progress
- The Challenges of Duchenne Muscular Dystrophy (DMD): A Deep Dive into Delays, Costs, and Progress
- The Role of Data in Advancing DMD Research
- The Crushing Financial Burden of Duchenne Muscular Dystrophy (DMD)
- High R&D Costs and Limited Competition
- Future Directions: A Path Towards Hope
- Reducing Drug Development Costs
- Why is Duchenne Muscular Dystrophy (DMD) Mostly Affecting Boys?
- Why is Duchenne Muscular Dystrophy (DMD) Progressive?
- Why is Duchenne Muscular Dystrophy (DMD) Not Entirely Curable?
- Why is Muscular Dystrophy Caused and How Does It Develop?
- Why is Duchenne Muscular Dystrophy (DMD) More Common in Males?
- Timeline of Duchenne Muscular Dystrophy (DMD): A History of Discovery and Drug Development
- Duchenne in 21st Century: Modern Therapeutic Advances
- Duchenne in 2020–Present: Innovations in Gene Therapy
- Challenges in Drug Development
- Duchenne Breakthroughs in the 2000s: The First Duchenne Muscular Dystrophy (DMD) Drugs
- Patient Involvement in Clinical Trials
- Looking Ahead: The Future of Duchenne Muscular Dystrophy (DMD) Treatment
- Conclusion: A Legacy of Perseverance and Hope
Duchenne Muscular Dystrophy and Its Long Road to Progress
Duchenne Muscular Dystrophy (DMD) is a rare genetic disorder that was first identified in the 19th century by French neurologist Guillaume Duchenne.
Characterized by progressive muscle degeneration, DMD affects approximately 1 in 3,500 male births worldwide.
Despite its early discovery in 1860, the first effective therapy only emerged in the early 2000s, reflecting the challenges in addressing rare diseases.
This blog delves into the history of DMD research and drug development, highlighting the persistence of scientists, pharmaceutical companies, and advocacy groups in combating this life-threatening condition.
Discover the latest advancements in Duchenne Muscular Dystrophy treatment in 2025, offering hope for better management and improved outcomes for patients.
We also explore the significant hurdles, such as
- the high costs of treatment,
- and the hope brought by modern advances in genetic therapies
- by examining the scientific breakthroughs and ongoing challenges.
This timeline sheds light on the journey to understand and treat Duchenne Muscular Dystrophy (DMD), emphasizing the resilience and collaboration that continue to drive progress.

The Challenges of Duchenne Muscular Dystrophy (DMD): A Deep Dive into Delays, Costs, and Progress
A Historical Perspective: Why Did It Take So Long?
The journey to develop treatments for Duchenne Muscular Dystrophy (DMD) highlights the technological and scientific hurdles of past centuries.
First described in the 19th century by Guillaume Duchenne, the disease’s genetic basis was not identified until 1986. The scientists pinpointed the dystrophin gene – the largest human gene responsible for encoding the muscle-protecting protein dystrophin.
This delay underscores the limitations of early genetics research and the inherent complexity of addressing rare diseases. Effective drug discovery required
- advancements in understanding disease mechanisms,
- access to modern research tools,
- and substantial funding—resources that were unavailable for much of this time.
The Role of Data in Advancing DMD Research
Modern breakthroughs in DMD research owe much to robust data collection and patient involvement. Patient registries like the TREAT-NMD Global Database have played a pivotal role by gathering:
- Genetic Data: Identifying mutations in the dystrophin gene to stratify patients for clinical trials.
- Clinical Data: Tracking disease progression to determine therapeutic windows.
- Biomarkers: Using markers such as creatine kinase levels to measure disease severity and treatment efficacy.
This data-driven approach has enabled the design of targeted therapies and streamlined clinical trials, significantly accelerating progress.
The Crushing Financial Burden of Duchenne Muscular Dystrophy (DMD)
Costs Faced by Families
Families living with Duchenne Muscular Dystrophy (DMD) grapple with overwhelming expenses, including:
- Medication: Monthly costs often run into thousands of dollars.
- Therapies: Physical and occupational therapies add to the financial strain.
- Equipment: Assistive devices like wheelchairs and braces incur additional expenses.
Beyond these direct costs, families face lost wages, increased household expenses, and emotional stress, creating a compounded financial toll. Crowdfunding has become the biggest boon for families who are endeavouring to get treatment for their loved ones. In India, ImpactGuru.com is the leading crowdfunding platform that caters to families raising funds for DMD.
Global Prevalence and Economic Impact
Duchenne Muscular Dystrophy (DMD) affects 1 in 3,500 male births worldwide, with treatment costs magnified in regions with limited resources.
The small patient population and high research investments lead to elevated prices as pharmaceutical companies recoup their development costs.
High R&D Costs and Limited Competition
Complex Research and Expensive Clinical Trials
Developing Duchenne Muscular Dystrophy (DMD) drugs is challenging due to the genetic complexity of the disease. Clinical trials, while essential, are lengthy and costly. The high failure rate of drug development also contributes to the steep price tags of successful therapies.
Additional Cost Drivers in Manufacturing and Distribution
Producing Duchenne Muscular Dystrophy (DMD) drugs involves specialized manufacturing processes, stringent quality standards, and specific storage conditions—all of which add to costs.
The global supply chain and handling requirements further increase expenses, ultimately impacting affordability for patients.
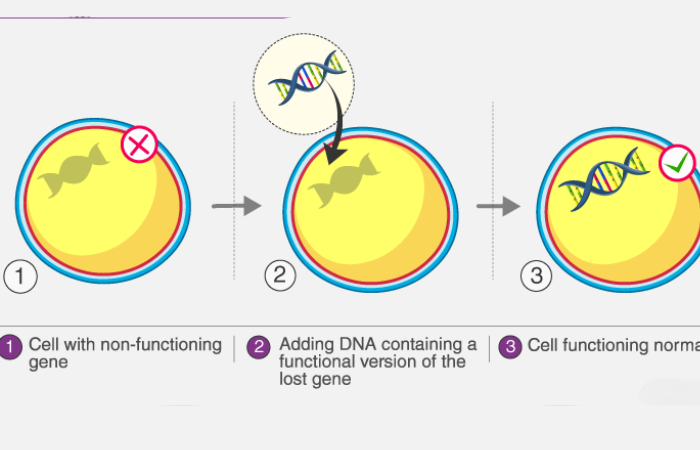
Future Directions: A Path Towards Hope
Innovations in Gene Therapy and Precision Medicine
Emerging types of therapy, such as gene therapy, offer the potential for long-term solutions. While still in development, these advancements could revolutionize treatment and reduce costs in the future.
Reducing Drug Development Costs
Improving efficiency in research and fostering collaborations across the pharmaceutical industry can lower development expenses. By sharing resources, stakeholders can mitigate risks and expedite breakthroughs.
The Importance of Continued Collaboration
Ongoing research, combined with cooperation among scientists, medical professionals, and advocacy groups remains vital. These efforts promise to bring innovative treatments to the forefront while making them more accessible and affordable.

Why is Duchenne Muscular Dystrophy (DMD) Mostly Affecting Boys?
Duchenne Muscular Dystrophy (DMD) primarily affects boys because it is caused by mutations in the dystrophin gene, located on the X chromosome. Boys inherit only one X chromosome (from their mother) and one Y chromosome (from their father).
If the dystrophin gene on their single X chromosome is mutated, they do not have a backup copy to produce functional dystrophin protein, leading to the development of DMD. In contrast, females have two X chromosomes, meaning a mutation in one copy of the dystrophin gene is usually compensated by the second, healthy copy.
This is why Duchenne Muscular Dystrophy (DMD) is rare in females, who are typically carriers without severe symptoms.
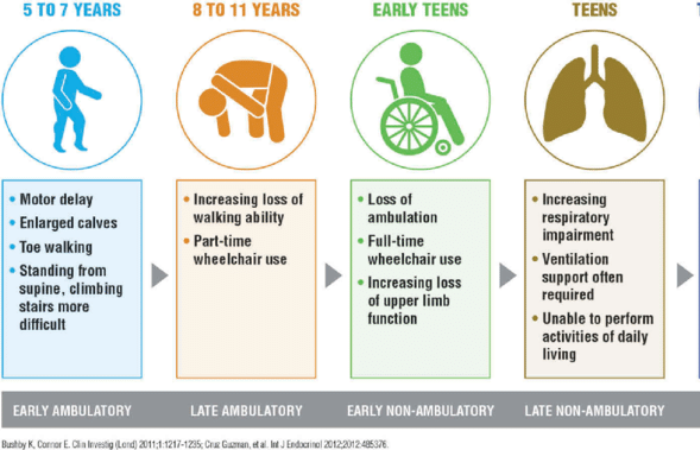
Why is Duchenne Muscular Dystrophy (DMD) Progressive?
Duchenne Muscular Dystrophy (DMD) is described as progressive because it involves a gradual weakening and degeneration of muscles over time. The absence of dystrophin, a protein critical for maintaining muscle cell integrity, causes muscle fibres to become fragile and prone to damage during normal use.
Over time, repeated cycles of muscle injury and insufficient repair result in the replacement of muscle tissue with fat and scar tissue, further impairing muscle function.
This ongoing deterioration underscores why Duchenne Muscular Dystrophy (DMD) is progressive and why early intervention is critical to managing symptoms.
Why is Duchenne Muscular Dystrophy (DMD) Not Entirely Curable?
- The lack of a cure for Duchenne Muscular Dystrophy (DMD) lies in the complexity of its genetic basis and the challenges of restoring or compensating for the dystrophin protein.
- Dystrophin is encoded by the largest human gene, making it difficult to repair or replace using conventional gene therapy techniques.
- Current treatments, such as corticosteroids or exon-skipping therapies, focus on slowing disease progression rather than curing it.
- Additionally, gene-editing technologies like CRISPR/Cas9 are still in experimental stages, and their long-term safety and efficacy remain uncertain.
- This highlights why Duchenne Muscular Dystrophy (DMD) is not curable at present.
Why is Muscular Dystrophy Caused and How Does It Develop?
Muscular dystrophy, including Duchenne Muscular Dystrophy (DMD), is caused by genetic mutations that interfere with the production or function of proteins essential for muscle health.
In DMD, the dystrophin gene mutation disrupts the synthesis of the dystrophin protein, leading to muscle damage and loss.
Individuals with DMD have muscle fibres that cannot withstand the mechanical stress of contraction, resulting in tears in the muscle cell membrane and leakage of intracellular contents.
Over time, this process leads to inflammation, cell death, and the progressive loss of muscle tissue. This explains why muscular dystrophy is caused and why it results in such devastating effects.
Why is Duchenne Muscular Dystrophy (DMD) More Common in Males?
The higher prevalence of Duchenne Muscular Dystrophy (DMD) in males is linked to its inheritance pattern.
Since the dystrophin gene mutation resides on the X chromosome, males with the mutation are more likely to develop the disease due to the absence of a second X chromosome to counteract the defect.
Females, on the other hand, are typically carriers because their second X chromosome usually contains a functional dystrophin gene, offering protection against the disease.
This genetic explanation underscores why Duchenne Muscular Dystrophy (DMD) is more common in males and why it remains a devastating diagnosis for boys and their families
Timeline of Duchenne Muscular Dystrophy (DMD): A History of Discovery and Drug Development
Duchenne in the 19th century
- 1836: The condition now known as Duchenne Muscular Dystrophy was first described by Sir Charles Bell, who noted a muscle-wasting disorder in young boys.
- 1861: French neurologist Guillaume-Benjamin Duchenne conducted the first detailed clinical description of the disease. His work, published in 1868, gave the disorder its name, Duchenne Muscular Dystrophy.
Duchenne in the 20th Century
- 1950s: The Muscular Dystrophy Association (MDA) was established in the U.S., driving research funding and raising public awareness about muscular dystrophies, including Duchenne Muscular Dystrophy (DMD).
- 1960s: Advances in genetics linked Duchenne Muscular Dystrophy (DMD) to the X chromosome, explaining why the disease primarily affects boys. However, the specific gene responsible remained unidentified, hampering therapeutic developments.
- 1986: Dr. Louis Kunkel and his team at Boston Children’s Hospital identified the dystrophin gene, marking a major milestone. The absence of dystrophin protein was found to cause muscle degeneration in Duchenne Muscular Dystrophy (DMD) patients.
Duchenne in 1980s–1990s: Early Drug Development Challenges
- With the dystrophin gene identified, researchers began exploring potential therapies, including gene replacement and steroids.
- 1980s: Corticosteroids, like prednisone, were shown to improve muscle strength and slow progression temporarily. However, long-term side effects limited their use.
- 1990s: Attempts at gene therapy faced challenges, including difficulty delivering the large dystrophin gene into muscle cells and immune system reactions.
Duchenne in 21st Century: Modern Therapeutic Advances
The new millennium saw accelerated progress in understanding Duchenne Muscular Dystrophy (DMD) and significant breakthroughs in treatment research.
2000–2010: Exon Skipping and Advocacy
- 2000: Exon skipping emerged as a promising approach. This technique uses synthetic molecules, such as antisense oligonucleotides (AONs), to bypass faulty parts of the dystrophin gene and restore partial dystrophin production.
- 2003: Sarepta Therapeutics (formerly AVI BioPharma) began early research into exon-skipping drugs for Duchenne Muscular Dystrophy (DMD).
- 2004: Parent Project Muscular Dystrophy (PPMD), a patient advocacy group, expanded its role in funding research and influencing drug development policies.
- 2007: Preclinical studies demonstrated the potential of exon-skipping therapies like eteplirsen to address certain mutations in the dystrophin gene.
Duchenne in 2010–2020: Drug Approval for Exon Skipping
- 2013: The Duchenne Registry was launched to connect patients, researchers, and drug developers, fostering collaboration.
- 2016: Sarepta Therapeutics’ Exondys 51 (eteplirsen) became the first FDA-approved exon-skipping therapy for Duchenne Muscular Dystrophy (DMD). It marked a historic moment but faced controversy due to limited efficacy data.
- 2017: PTC Therapeutics received FDA approval for Emflaza (deflazacort), a corticosteroid specifically for Duchenne Muscular Dystrophy (DMD), offering fewer side effects compared to prednisone.
- 2019: Solid Biosciences faced challenges with its gene therapy program after clinical trial setbacks, highlighting the complexity of gene-based treatments.
Duchenne in 2020–Present: Innovations in Gene Therapy
A shift toward cutting-edge gene editing and gene-replacement technologies has made the current era remarkable for the treatment of Duchenne.
- 2021: Pharmaceutical companies like Pfizer and Sarepta Therapeutics both advanced their micro-dystrophin gene therapies into clinical trials. These therapies aim to introduce a smaller but functional version of dystrophin into muscle cells.
- 2023: Sarepta Therapeutics’ Elevidys (SRP-9001) received FDA approval as the first gene therapy for Duchenne DMD, providing hope for a long-term solution. However, questions remain about durability and accessibility.
- 2024: Ongoing CRISPR-Cas9 trials offer the potential to correct dystrophin mutations at the DNA level, representing a revolutionary approach in Duchenne Muscular Dystrophy (DMD) treatment.
Challenges in Drug Development
Developing therapies for Duchenne Muscular Dystrophy (DMD) has been fraught with challenges:
- Complexity of the Dystrophin Gene: The dystrophin gene is one of the largest in the human genome, making it difficult to manipulate and deliver effectively.
- Diversity of Mutations: Over 4,000 known mutations can cause Duchenne Muscular Dystrophy (DMD), complicating the development of universal treatments.
- Regulatory Hurdles: Limited patient populations and variability in disease progression have made it difficult to conduct robust clinical trials.
- High Costs: Gene therapy and exon-skipping drugs are expensive to develop and administer, raising concerns about accessibility.
- Immune Response: Delivering therapies via viral vectors can trigger immune reactions, hindering their effectiveness.
This comprehensive data allowed researchers to design targeted therapies and streamline clinical trials.
Duchenne Breakthroughs in the 2000s: The First Duchenne Muscular Dystrophy (DMD) Drugs
The early 2000s marked a turning point in Duchenne Muscular Dystrophy (DMD) treatment with the advent of exon-skipping therapies.
These therapies use antisense oligonucleotides (ASOs) to skip over faulty exons during gene transcription, partially restoring dystrophin production. Key milestones include:
- Eteplirsen (Exondys 51): Approved by the FDA in 2016, it was the first drug to target specific Duchenne Muscular Dystrophy (DMD) mutations.
- Golodir Sen and Victor Larsen: Subsequent exon-skipping drugs expanded treatment options for additional mutations.
- Gene Therapy: Emerging approaches, such as micro-dystrophin gene therapy, aim to introduce functional versions of the dystrophin gene, offering hope for more comprehensive treatments.
Patient Involvement in Clinical Trials
Patients and their families have been at the forefront of Duchenne Muscular Dystrophy (DMD) advancements. Their participation in clinical trials provided essential data to evaluate drug efficacy and safety. Challenges include:
- Recruitment: Ensuring sufficient trial participants for a rare disease is difficult.
- Ethical Considerations: Balancing experimental treatments with patient safety requires rigorous oversight.
- Longitudinal Studies: Tracking patients through the years provides valuable insights but demands sustained commitment.
Despite these hurdles, patient involvement proves invaluable. Their contributions not only advance treatment options but also foster a community united by shared goals.
Looking Ahead: The Future of Duchenne Muscular Dystrophy (DMD) Treatment
The journey to finding a drug for DMD underscores the importance of collaboration among researchers, patients, and advocates. As gene-editing technologies like CRISPR/Cas9 and stem cell therapies advance, the future holds promise for curative treatments.
Furthermore, ongoing data collection through registries and real-world evidence studies will continue to refine therapeutic approaches and ensure that all patients benefit from scientific progress.
Conclusion: A Legacy of Perseverance and Hope
The Long Road to Progress
The journey to develop treatments for Duchenne Muscular Dystrophy (DMD) has been arduous, marked by scientific ingenuity, setbacks, and breakthroughs.
Pharmaceutical organizations like Sarepta Therapeutics, Pfizer, and advocacy groups such as Parent Project Muscular Dystrophy (PPMD) have played pivotal roles in advancing research and ensuring the voices of patients and families are heard.
While significant strides have been made, the ultimate goal – a cure for Duchenne Muscular Dystrophy (DMD) – remains on the horizon.
The Challenge of High Costs
Understanding the high costs of Duchenne Muscular Dystrophy (DMD) treatments is essential for families navigating care options.
The financial burden may feel overwhelming, but avenues for support exist, including advocacy for affordable therapies and informed decision-making.
Efforts to reduce costs and increase accessibility are crucial to ensuring that all affected families can benefit from medical advancements.
Looking to the Future
Emerging technologies, such as CRISPR and enhanced gene therapies, offer renewed hope for the future of Duchenne Muscular Dystrophy (DMD) treatment.
These innovations bring us closer to the ultimate goal of transforming (DMD) from a life-threatening condition into a manageable, or even curable, disorder.
A Testament to Resilience
The delay in developing effective Duchenne Muscular Dystrophy (DMD) treatments reflects the complexities of scientific discovery and the challenges of addressing rare diseases.
However, the progress made since the 2000s underscores the power of data, patient advocacy, and collaboration.
The story of Duchenne Muscular Dystrophy (DMD) drug development is one of perseverance and innovation – a powerful reminder of what can be achieved when communities unite to tackle seemingly insurmountable challenges.
Duchenne Muscular Dystrophy (DMD) is a genetic disorder that causes progressive muscle weakness and degeneration, primarily affecting young boys. It is caused by mutations in the dystrophin gene, leading to a lack of the protein that protects muscle cells.
Traditionally, treatment for DMD has focused on managing symptoms with physical therapy, steroids, and sometimes surgery. However, these treatments only slow progression and don’t cure the condition.
Gene therapy aims to correct or replace the defective dystrophin gene responsible for DMD. This innovative approach holds promise for halting or reversing muscle degeneration in DMD patients, offering new hope for better outcomes.
In 2025, gene therapy for Duchenne Muscular Dystrophy is showing promising results in clinical trials, with some treatments demonstrating the ability to restore or improve muscle function. While it’s still evolving, these therapies offer significant hope for the future.
Like any advanced treatment, gene therapy for DMD carries risks such as immune reactions or unintended side effects. However, ongoing research aims to minimize these risks while improving the therapy’s safety and efficacy.
Sources:
NINDS – Muscular Dystrophy
Wikipedia – Duchenne muscular dystrophy
Parent Project Muscular Dystrophy












